Tinlarebant
PHASE 3
AFFILIATIONS


HOPE TO BLINDNESS
For Geographic Atrophy & Stargardt Disease
Tinlarebant, or LBS-008, if approved, would provide a novel treatment option. Tinlarebant is a novel, once-a-day oral therapy which is intended to reduce the accumulation of toxins in the eye that cause Stargardt Disease (STGD1) and contribute to Geographic Atrophy (GA), or advanced dry AMD. These toxins are by-products of the visual cycle, which is dependent on the supply of vitamin A (retinol) to the eye. Tinlarebant works by reducing and maintaining levels of serum retinol binding protein 4 (RBP4), the sole carrier protein for retinol transport from the liver to the eye. By modulating the amount of retinol entering the eye, Tinlarebant reduces the formation of these toxins. In clinical trials, Tinlarebant has demonstrated its target specificity and potency that we believe could be clinically meaningful to treat (STGD1) patients and GA patients.
Tinlarebant is a potent, orally administered small molecule RBP4 antagonist that has been specifically designed to reduce the delivery of retinol to the eye as a therapeutic approach towards reducing the accumulation of cytotoxic bisretinoids, preserving the integrity of retinal tissues, and ultimately slowing or preventing loss of vision. The delivery of retinol to the RPE requires RBP4 and the RPE expresses a specific RBP4 receptor (STRA6) to regulate vitamin A uptake. Other extrahepatic tissues do not require delivery of retinol bound to RBP4 and do not express the RBP4 receptor. These tissues are able to take up vitamin A bound to non-specific carriers such as lipoproteins, triglycerides, and albumin.
Tinlarebant was selected by the National Institute of Health (NIH) Blueprint Neurotherapeutics Network, (BPN) in 2011 as a promising drug candidate for treating dry AMD. The BPN was launched in 2004 to foster small-molecule neurotherapeutic development, bringing together a unique blend of grant dollars, industry-standard scientific expertise, and contract resources under a milestone-driven cooperative agreement program.
Additionally, the mechanism of action utilized by Tinlarebant has been recognized and recommended as a priority for clinical development in both STGD1 and dry AMD in a systematic review published by the U.K. National Institute for Health Research, or the NIHR, in 2018. The NIHR screened 7,948 articles in 2018 for its systematic review on treatments for dry AMD and STGD1. Its principal findings included that research focus should be at earlier stages in both diseases (before vision is impaired) and that the most promising treatments for both diseases appear to be prevention of lipofuscin and bisretinoid accumulation. Therefore, the NIHR recommended the mechanism of RBP4 inhibition, which is utilized by Tinlarebant, as a promising treatment in dry AMD and STGD1.
We have completed a two-year Phase 2 study of Tinlarebant in adolescent STGD1 subjects, and are conducting a Phase 3 study (DRAGON) and a Phase 2/3 study (DRAGON II) in adolescent STGD1 subjects and a Phase 3 study (PHOENIX) in subjects with GA. DRAGON study is a multi-center, randomized, double masked, placebo controlled study to evaluate the safety and efficacy of Tinlarebant in the treatment of adolescent STGD1 patients. DRAGON II trial is a combination of phase 1b open-label study to evaluate the pharmacokinetics and pharmacodynamics of Tinlarebant in Japanese adolescent STGD1 subjects and a phase 2/3, multicenter, double-masked, placebo-controlled, randomized study designed to evaluate the efficacy, safety and tolerability of Tinlarebant in adolescent STGD1 subjects. PHOENIX study is a multi-center, randomized, double‑masked, placebo-controlled study to evaluate the safety and efficacy of Tinlarebant in GA subjects. Tinlarebant has obtained Orphan Drug Designation in the United States, Europe, and Japan, and has been granted the Rare Pediatric Disease (RPD) designation and the Fast Track Designation in the US and Sakigake (Pioneer Drug) Designation in Japan.
DISEASE PROFILE
Geographic Atrophy
Dry age-related macular degeneration (dry AMD) is a leading cause of vision loss in older adults. Geographic Atrophy, or GA, is the advanced stage of dry AMD. Currently, there are no FDA approved orally administered treatments for GA and no FDA approved therapies for the other stages of dry AMD other than GA. There are an estimated 20 million AMD patients in the U.S. and over 196 million patients worldwide with an estimated global direct healthcare cost of US$255 billion.
Stargardt Disease
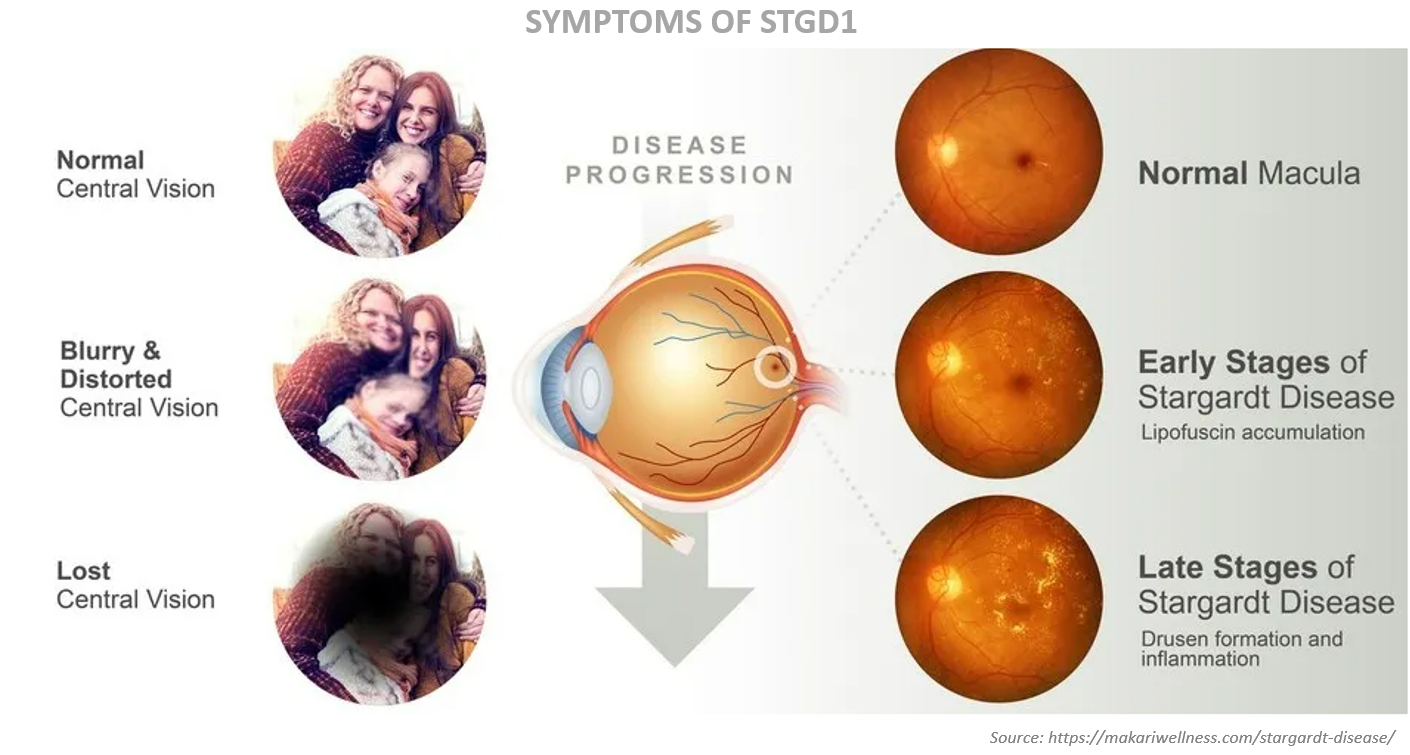
Stargardt disease (STGD1) is an inherited juvenile form of macular degeneration and currently, there is no approved treatment available. The disease is caused by a mutation in the ABCA4 gene, which leads to the accelerated formation and accumulation of toxic vitamin A by-products known as bisretinoids. The most prominent bisretinoid identified in human tissues is known as A2E (N-retinylidene-N-retinylethanolamine). The accumulation of A2E in ocular tissues causes progressive retinal cell death and permanent loss of vision. More than 500 mutations in the ABCA4 gene have been identified in STGD1 patients. Some STGD1 patients suffer severe visual impairment by the age of 20. The prevalence rate of STGD1 is estimated to be 1 in 10,000 people. Based on this estimate, approximately 30,000 US citizens are affected by STGD1. This estimate includes both adults and children.
MECHANISM OF ACTION
Preventing the Toxic Accumulation of Lipofuscin
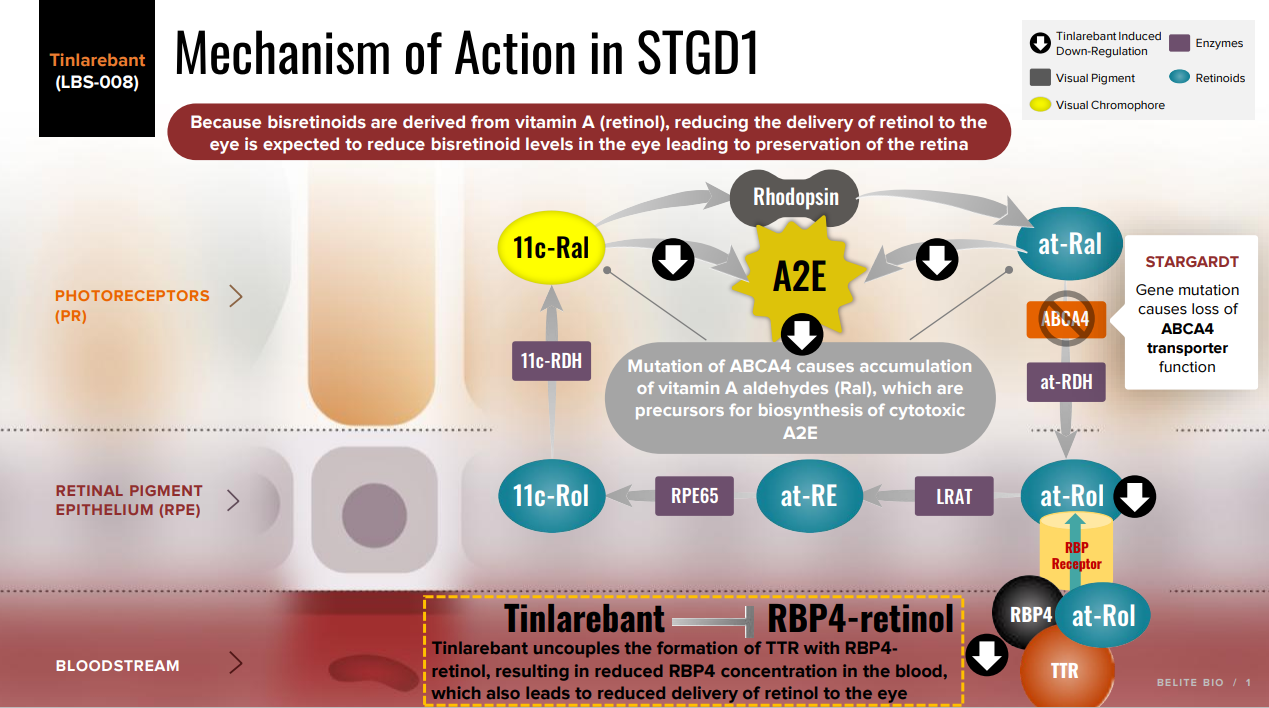
Both STGD1 and dry AMD are characterized by the early aberrant accumulation of lipofuscin and cytotoxic bisretinoids. The most abundant autofluorescent bisretinoid that has been identified in human lipofuscin is known as A2E (N-retinylidene-N-retinylethanolamine), a spontaneously formed complex comprised of two molecules of retinal and one molecule of ethanolamine. Investigations of the potential toxicity of A2E in cell-based assays and animal models have shown that this compound is highly toxic and can kill RPE cells in a concentration-dependent manner through myriad mechanisms. Because A2E and related bisretinoids are derived from vitamin A (i.e., they are by-products of normal visual cycle function), therapeutic approaches have focused on reducing levels of vitamin A (retinol) in the eye.
Visual Cycle
The processing of vitamin A (aka, retinol, or all-trans retinol) in the visual cycle begins with the delivery of circulating retinol to the RPE. The ternary complex of RBP4-retinol-TTR is presented to RBP4 receptors which are located on the basal surface of RPE. The RBP4-TTR vehicle serves to solubilize retinol and produce a large molecular size complex which resists elimination in the kidney. Upon entry into the RPE, retinol undergoes a series of enzymatic reactions resulting in generation of the visual chromophore, 11-cis retinal. The visual chromophore is delivered to the retina where it combines with opsin to form the light-sensitive visual pigment, rhodopsin. Photoactivation of rhodopsin liberates all-trans retinal which is transported out of the retina by the ABCA4 protein.